Gale Hawkins
Super Star Member
- Joined
- Sep 20, 2009
- Messages
- 11,759
- Location
- Murray, KY
- Tractor
- 1948 Allis Chambers Model B 1976 265 MF / 1983 JD 310B Backhoe / 1966 Ford 3000 Diesel / 1980 3600 Diesel
PV numbers continue to increase.
 www.pv-magazine.com
www.pv-magazine.com

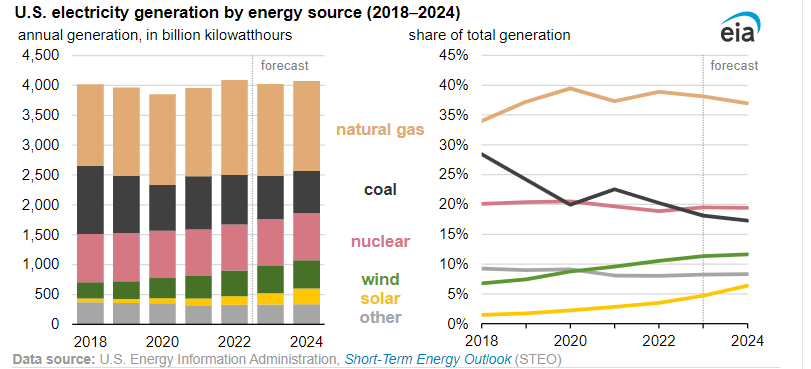
US to add 63 GW of PV by end 2024
Solar and wind generation are expected to reach 16% of the US grid’s supply this year, doubling the 2018 total, said the US Energy Information Administration (EIA). By 2024, renewables will account for more than one-quarter of electricity generation in the United States.
 ...no I'm not. A closed container, atmospheric air and a running mower in it. As the gasoline is burned "experts" say the weight increases.
...no I'm not. A closed container, atmospheric air and a running mower in it. As the gasoline is burned "experts" say the weight increases.