MinnesotaDaveChalmers
Platinum Member
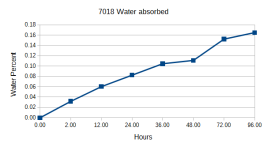
There are two reasons I can think of that ovens are maintained at higher temps. One is to vaporize light oils, like WD40, kerosene and diesel, which usually boil at about 400-500F. The other is to heat up the large masses of welding rods that get put in there as quickly as possible. If the oven were at 250F, it might take 8 hours to get 50# of rods to 250F. If the oven is at 500F, it only takes a few hours to get the rods to 250F. If water were the only contaminant in the rods, and no one was in a hurry, the oven could be kept at the boiling point of water.
Storage ovens are only approximately 250-300 degrees. This temp will not re-dry (re-condition) 7018 according to rod manufacturers.
It's the re-drying that is at the significantly higher temperatures. Lincoln's chart is 650-750 degrees for that.